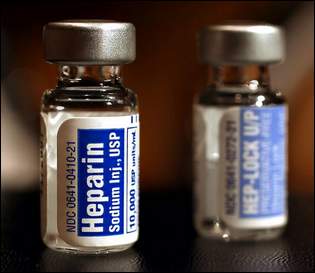
Counterfeit heparin may be responsible for at least 19 deaths in the United States and hundreds of allergic reactions. The news came from federal regulators yesterday that part of the anti-clotting drug made by Baxter was an unapproved ingredient from China that was altered to mimic the real thing. Baxter has pulled the drug from the market.
In exploring the potential liability of Baxter below, I first offer up this personal background: I previously represented Timothy Fagan, a 16 year-old New Yorker who had been injected with counterfeit Epogen after an emergency liver transplant. It was one of the few counterfeit drugs cases ever brought in this country, which gives me a unique perspective on the issues that may be encountered should suit be brought against Baxter. His experience was part of a 60 Minutes segment (as well as many other news reports) and featured in a book by Katherine Eban, Dangerous Doses. Legislation named for him, Tim Fagan’s Law, is pending in Congress. I have a page at my web site devoted to the subject (Counterfeit Drug Resource Page), have written on the subject here a number of times (though not in the last nine months), and spoken at pharmaceutical conferences on the subject.
Tim’s experience, like the one with Baxter’s heparin, resulted from problems in the pharmaceutical supply chain. For Tim, the counterfeiting took place after the drugs left the hands of drugmaker Amgen, and low dose vials were “uplabled” with counterfeit labels to appear to be 20x the strength, reportedly by a criminal gang in Florida. The vials were also mishandled, leading to apparent adulteration. Tracing how the drugs moved through a web of secondary wholesalers was a critical part of the investigation.
Baxter now faces a similar problem of supply chain management, though the problems exist upstream instead of downstream. The problems result from outsourcing critical manufacturing to others while also failing to verify the integrity of the product. Any investigation as to Baxter liability will no doubt turn on whether the company turned a blind eye to the product’s sourcing, perhaps because the price was so good.
Litigants will also face a critical set of problems that will arise in any drug counterfeiting case:
- The evidence was destroyed at the time it was injected or ingested and the packaging discarded.
- Doctors will generally assume that a failure to get better is the result of the underlying condition, not a counterfeit drug.
- Since the patient was already sick (or they wouldn’t be getting the drug), proving that death or further disability came from the counterfeit, as opposed to an underlying cardiac condition that they were perhaps being treated for, will represent a real causation issue even if you know the counterfeit was injected/ingested.
- The drug may not be trackable back to Baxter due to shoddy record keeping regarding the supply chain, which still contains loopholes that allow the “pedigree” of a drug to be washed by “authorized distributors of record” so that prior owners of the drug are unknown.
Litigants will start with an essential fact: It is a prohibited act to sell counterfeit drugs and Baxter appears to have done just that. Most lawyers refer to that as negligence per se.
Since Baxter appears to have committed that prohibited act (assuming the accuracy of press reports), it must therefore try to defend itself with a claim that the company owed no duty of care to the end-user, as there was no direct relationship between the two.
A savvy litigant will respond, however that the since the Food, Drug and Cosmetic Act prohibits introducing defective drugs into interstate commerce, it is not enough to say that Baxter simply didn’t know that this is what their sourcing companies were doing. The United States Supreme Court, in the little known 1975 case of US v. Park, has already stated that the Act “imposes not only a positive duty to seek out and remedy violations when they occur but also, and primarily, a duty to implement measures that will insure that violations will not occur.” The Act, according to the Court, punishes “neglect where the law requires care, or inaction where it imposes a duty.”
Since the measure of Baxter’s duty to the end-user will be measured by the foreseeable risk, and counterfeiting is not only a clearly foreseeable risk (see the links back at my resource page), but one that has received much attention lately due to counterfeits coming out of China in particular, the foreseeability issue is easily approached.
Thus, a plaintiff would argue that it is not whether Baxter owes its customers a duty of care, which has existed for decades, but rather, the scope of that duty.
The legal issues of whether a duty of care exists between manufacturer and consumer also might be addressed from the breach of warranty angle. In that respect, relief may exist (among other places) in its uniform commercial code. New York’s UCC 2-318, for example, provides that: “A seller’s warranty whether express or implied extends to any natural person if it is reasonable to expect that such person may use, consume or be affected by the goods and who is injured in person by breach of the warranty. “
Baxter counsel will argue, in essence, that the company deserves immunity, and will scratch around for any argument that fits that bill. Since any case will likely be faced with a motion to dismiss right away, it is critical that each of the potential issues be addressed in the complaint with proper allegations. There are no cookie cutter forms for this type of complaint, and each of the allegations and potential responses must be thought through and specifically tailored.
Update:
- Baltimore Sun says 21 deaths and 700 injured)
- Senator Edward Kennedy released a statement that said, in part:
“It is unacceptable that Americans have died and been seriously injured by what appears to be deliberate tampering. Whether this contaminant was introduced intentionally or by accident, the full force of the law must be brought to bear to bring those responsible to justice. To guard against future abuses, every drug manufacturer needs to inform FDA of where it sources its ingredients and what it is doing to ensure that these ingredients are pure and potent.”
General counterfeit drug links:
Counterfeit heparin links:
Update 3/27/08:
 The FDA revealed yesterday that the death toll from counterfeit heparin rose from 19 to 62 over the last 15 months starting in January 2007. I had previously discussed this issue in the context of Baxter’s potential liability for distributing counterfeit drugs (see, Counterfeit Heparin and Baxter Liability).
The FDA revealed yesterday that the death toll from counterfeit heparin rose from 19 to 62 over the last 15 months starting in January 2007. I had previously discussed this issue in the context of Baxter’s potential liability for distributing counterfeit drugs (see, Counterfeit Heparin and Baxter Liability).